Asid nikleyik viris grip/viris grip B ki seche nan frizè
Non pwodwi
HWTS-RT193-Twous Deteksyon Asid Nikleyik Viris Grip/Viris Grip B ki seche nan frizè (PCR fliyoresans)
Epidemyoloji
Selon diferans antijenik ki genyen ant jèn NP ak M yo, viris grip yo ka divize an kat kalite: viris grip A (IFV A), viris grip B (IFV B), viris grip C (IFV C) ak viris grip D (IFV D). Viris grip A a gen anpil lame ak serotip konplèks, epi li ka gaye atravè lame yo atravè rekombinasyon jenetik ak mitasyon adaptatif. Moun pa gen iminite dirab kont viris grip A a, kidonk moun tout laj jeneralman sansib. Viris grip A a se patojèn ki pi enpòtan ki lakòz pandemi grip yo. Pou viris grip B a, li gaye sitou nan ti zòn epi kounye a li pa gen okenn soutip. Enfeksyon imen yo sitou koze pa viris grip ki soti nan liyaj B/Yamagata oswa B/Victoria. Pami ka grip konfime chak mwa nan 15 peyi nan rejyon Azi Pasifik la, to dyagnostik viris grip B a varye ant 0 ak 92%. Kontrèman ak viris grip A a, sèten gwoup moun, tankou timoun ak granmoun aje, ki vilnerab a viris grip B a, ki ka fasilman lakòz konplikasyon, ki poze plis fado pou sosyete a pase viris grip A a.
Paramèt teknik
| Depo | 2-28℃ |
| Dire lavi etajè | 12 mwa |
| Kalite echantiyon | Tiyo pou gòj |
| Ct | IFV A& rsquo;IFVB Ct≤35 |
| CV | <5.0% |
| LoD | 200 Kopi/mL |
| Espesifikite | Kwa-reyaktivite: Pa gen okenn kwa-reyaktivite ant twous la ak Bocavirus, Rhinovirus, Cytomegalovirus, Viris respiratwa syncytial, Viris Parainfluenza, Viris Epstein-Barr, Viris èpès senp, Viris varisèl-zostèr, Viris oreyon, Enterovirus, Viris lawoujòl, metapneumovirus imen, Adenovirus, kowonaviris imen, nouvo kowonaviris, SARS-CoV, MERS-CoV, Rotavirus, Norovirus, Chlamydia pneumoniae, Mycoplasma pneumoniae, Streptococcus pneumoniae, Klebsiella pneumoniae, Streptococcus pyogenes, Legionella, Pneumocystis jirovecii, Haemophilus influenzae, Bordetella pertussis, Staphylococcus aureus, Mycobacterium tuberculosis, Neisseria gonorrhoeae, Candida albicans, Candida glabrata, Aspergillus fumigatus, Cryptococcus neoformans, Streptococcus salivarius, Moraxella catarrhalis. Lactobacillus, Corynebacterium ak ADN jenomik imen. Tès entèferans: Yo te chwazi Mucin (60mg/mL), san moun (50%), Phenylephrine (2 mg/mL), Oxymetazoline (2mg/mL), Klori sodyòm (20mg/mL) avèk 5% konsèvatif, Beclomethasone (20mg/mL), Dexamethasone (20mg/mL), Flunisolide (20μg/mL), Triamcinolone (2mg/mL), Budesonide (1mg/mL), Mometasone (2mg/mL), Fluticasone (2mg/mL), Histamine hydrochloride (5 mg/mL), Benzocaine (10%), Menthol (10%), Zanamivir (20mg/mL), Peramivir (1mg/mL), Mupirocin (20mg/mL), Tobramycin (0.6mg/mL), Oseltamivir (60ng/mL), Ribavirin (10mg/L) pou tès entèferans lan, epi rezilta yo te montre ke sibstans ki entèfere yo nan konsantrasyon ki anwo yo pa te gen okenn reyaksyon entèferans ak rezilta tès twous la. |
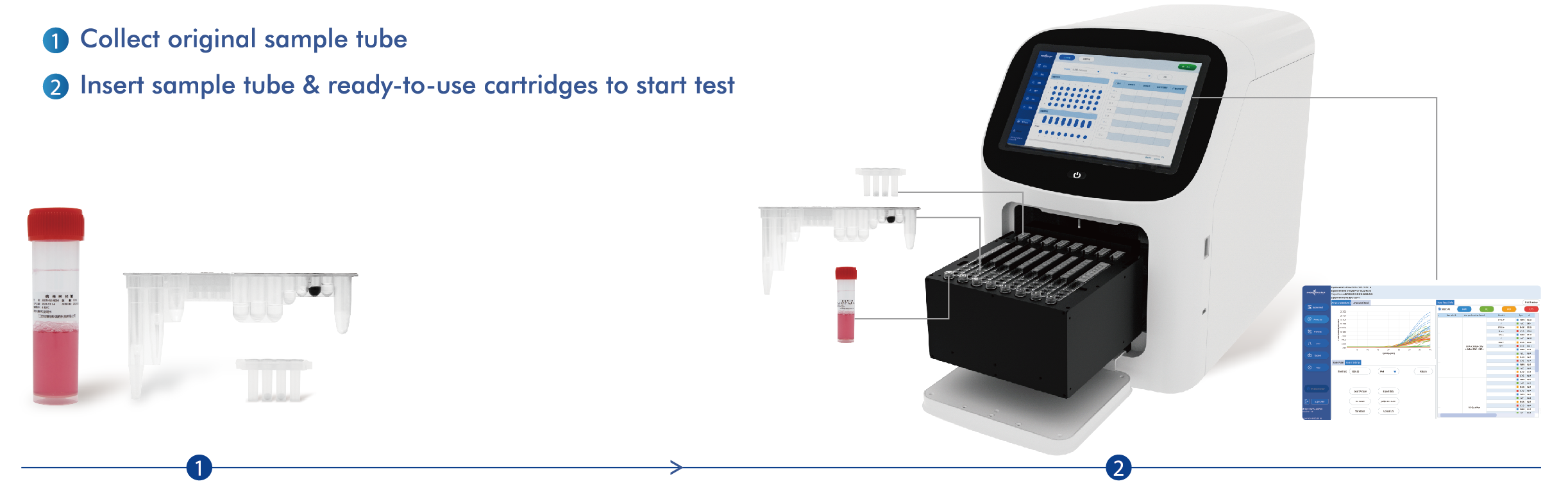
| Enstriman Aplikab yo | Aplikab pou reyaktif tès Tip I: Sistèm PCR an tan reyèl Applied Biosystems 7500 yo, Sistèm PCR an tan reyèl SLAN-96P (Hongshi Medical Technology Co., Ltd.). Aplikab pou reyaktif tès Tip II: EudemonTM AIO800 (HWTS-EQ007) pa Jiangsu Macro & Micro-Test Med-Tech Co., Ltd. |
Koule Travay
PCR konvansyonèl
Yo rekòmande Macro & Micro-Test General DNA/RNA Kit (HWTS-3019) (ki ka itilize avèk Macro & Micro-Test Automatic Nucleic Acid Extractor (HWTS-3006C, (HWTS-3006B) pa Jiangsu Macro & Micro-Test Med-Tech Co., Ltd.) pou ekstraksyon echantiyon an epi etap ki vin apre yo ta dwe fèt an akò strik avèk IFU Kit la.
Machin tout-an-yon AIO800