SARS-CoV-2/grip A/grip B
Non pwodwi
HWTS-RT148-SARS-CoV-2/grip A/grip B twous deteksyon konbine asid nikleyè (PCR fliyoresan)
Chèn
| Non chanèl | PCR-Mix 1 | PCR-Mix 2 |
| FAM Channel | ORF1ab jèn | IVA |
| VIC/HEX Channel | Kontwòl entèn | Kontwòl entèn |
| CY5 Chèn | N jèn | / |
| Chèn ROX | E jèn | IVB |
Paramèt teknik
| Depo | -18℃ |
| Etajè-lavi | 12 mwa |
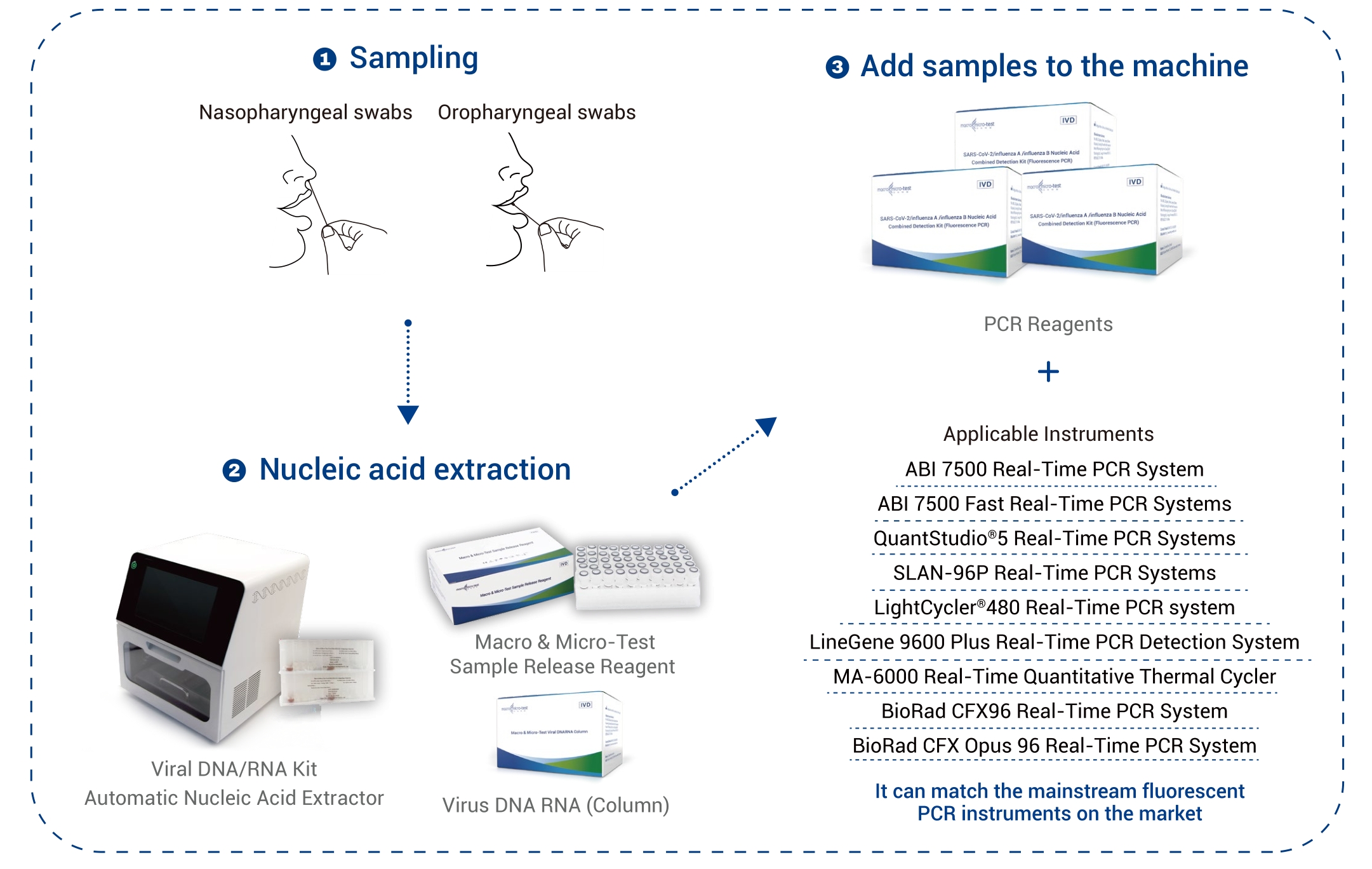
| Kalite echantiyon | prelèvman nazofarinj ak prelèvman oropharyngeal |
| Sib | Twa sib SARS-CoV-2 (jen Orf1ab, N ak E)/grip A/grip B |
| Ct | ≤38 |
| CV | ≤10.0% |
| LoD | SARS-CoV-2: 300 Kopi/mL viris grip A: 500 Kopi/mL viris grip B: 500 Kopi/mL |
| Espesifik | a) Rezilta tès kwaze yo te montre twous la konpatib ak coronavirus imen SARSr-CoV, MERSr-CoV, HcoV-OC43, HcoV-229E, HcoV-HKU1, HCoV-NL63, viris respiratwa syncytial A ak B, viris parainfluenza 1, 2 ak 3, rhinovirusA, B ak C, adenovirus 1, 2, 3, 4, 5, 7 ak 55, metapneumovirus imen, enterovirus A, B, C ak D, viris poumon sitoplasmik imen, viris EB, viris lawoujòl Sitomgalovirus imen, rotaviris, noroviris, viris malmouton, viris varisèl zoster, Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella, koklich, Haemophilus influenzae, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, Klebsiella pneumoniae, Candidatuberkulosis, Candidatubactebacteria ta Pa te gen okenn reyaksyon kwaze ant Pneumocystis yersini ak Cryptococcus neoformans. b) Kapasite kont entèferans: chwazi mucin (60mg/mL), 10% (V/V) san moun, diphenylephrine (2mg/mL), hydroxymethylzoline (2mg/mL), klori sodyòm (ki gen konsèvasyon) (20mg/mL), beclomethasone (20mg/mL), dexamethasone (20mg/mL), flunizon (20μg/mL), triamcinolone acetonide (2mg/mL), budesonide (2mg/mL), mometasone (2mg/mL), fluticasone (2mg/mL), histamine hydrochloride (5 mg/mL), α-Interferon (800IU/mL), zanamivir (20mg/mL), ribavirin (10mg/mL), oseltamivir (60ng/mL), pramivir (1mg/mL), lopinavir (500mg/mL). ), ritonavir (60mg/mL), mupirocin (20mg/mL), azithromycin (1mg/mL), ceprotene (40μg/mL) Meropenem (200mg/mL), levofloxacin (10μg/mL) ak tobramycin (0.6mg/mL) .Rezilta yo te montre ke sibstans ki entèfere nan konsantrasyon ki anwo yo pa te gen okenn repons entèferans nan rezilta deteksyon patojèn yo. |
| Enstriman ki aplikab | Applied Biosystems 7500 an tan reyèl sistèm PCR Applied Biosystems 7500 rapid an tan reyèl sistèm PCR SLAN ®-96P an tan reyèl sistèm PCR QuantStudio™ 5 Sistèm PCR an tan reyèl LightCycler®480 an tan reyèl sistèm PCR LineGene 9600 Plus an tan reyèl sistèm deteksyon PCR MA-6000 an tan reyèl Quantitative Thermal Cycler BioRad CFX96 an tan reyèl sistèm PCR BioRad CFX Opus 96 an tan reyèl sistèm PCR |
Solisyon PCR total